What Holds The Nucleus Together
Hither's what I would telephone call the One Sentence Summary Of Chemistry. If you acquire just ane affair most chemistry, learn this.
"Opposite charges attract, like charges repel".
We'll go into a lot more than item over the adjacent few posts, just let's get started by thinking of some examples.
- positive charges attract negative charges – Two examples here. Think of the negatively charged electron that orbits the positively charged nucleus. Or sodium chloride, which is equanimous of positively charged sodium ions held together with negatively charged chlorine ions. Positive attracts negative. Bank check.
- Negative charges repel negative charges. You may remember that the shape of a molecule depends on the number of bonds/electron pairs around an atom. Why is water bent, for example? That'south because the oxygen lone pairs each take up space, and the electron pairs repel until they are the maximum distance autonomously. That'southward also why a molecule like methane is tetrahedral and not square planar.
- Positive charges repel positive charges. It'due south harder to recollect of examples here, but hither's one. It'south relatively easy to add together a proton to water to give [H3O]+, but very difficult to protonate that further to give [H4O]two+ due to repulsion.
It's this last ane which seems weird. If information technology's so hard to make [H4O]2+, for instance, how is information technology that the nucleus holds together, since information technology'south substantially made of positively charged protons with a few neutrons thrown in.? It'south specially weird when you consider that these positive charges are distributed over such a short distance.
Permit'due south take a step dorsum to await at the Really Big Picture show to discover out.
The Really Big Film
Physicists have identified iv fundamental forces in nature that are responsible for all the phenomena we observe. They are
- gravity, which acts at long range, and is attractive (but weak)
- electromagnetism – responsible for the beliefs of electric and magnetic fields.
- the weak nuclear force, responsible for certain types of nuclear decay (when a neutron breaks downwards into a proton and an electron, that's the weak nuclear force at piece of work).
- and so we come up to the potent nuclear force, which acts at very brusk range and is attractive.
The forces vary in strength over many, many orders of magnitude (getting the extremely weak gravitational force to fit into a Theory of Everthing, in particular, has been driving physicists basics for decades).
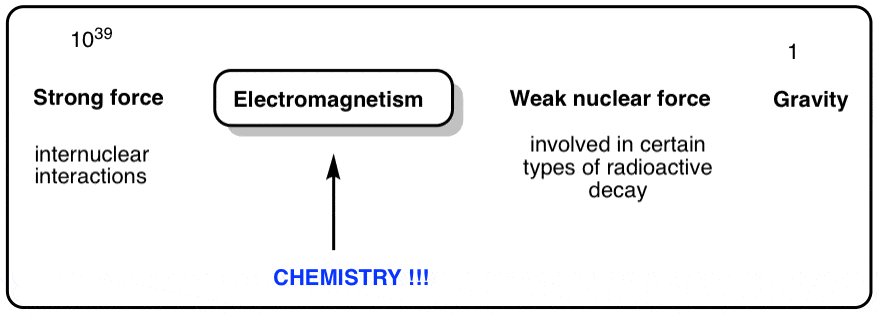
Two observations:
Chemistry is predominantly about electromagnetism . The study of chemistry is largely the study of electrons and how they flow between atoms. In chemical science, we're essentially just looking at electrons and nuclei as point charges, and treating the chemic bonds that course between them every bit electrostatic phenomena. Furthermore, the electromagnetic fields accompanied by these charges can collaborate with radiation with wavelengths all across the electromagnetic spectrum, giving ascension to the huge variety of spectroscopic techniques we have developed to help give u.s. clues as to the structure of atoms and molecules.
What about the other forces in chemistry? Gravity is irrelevant – compared to the electromagnetic force, it'due south a rounding error at the 30th decimal identify, essentially. The weak nuclear force doesn't much come into play either, peculiarly when it comes to the meat and potatoes organic chemical science we're going to be discussing. What nigh the potent nuclear forcefulness? It has one – and just one – of import part in the chemistry we're going to discuss, and that's information technology.
What holds the nucleus together? The stiff nuclear force. At extremely short range, information technology is stronger than electrostatic repulsion, and allows protons to stick together in a nucleus even though their charges repel each other. Remember that the size of the nucleus is really small compared to the size of an atom. Since it operates but over distances comparable with the bore of an cantlet, it isn't felt at longer distances, and for the purposes of chemistry, we ignore it.
When information technology comes to the stiff force, there is a pause-fifty-fifty point. Physicists take observed that the nucleus becomes increasingly more stable upon the add-on of nucleons upwards to iron-56. Then, as successive protons are added, the nucleus gets more than and more unstable, due to the electrostatic repulsion outweighing the attractive nuclear force. Once you get upward to really high atomic numbers, the nucleus becomes unstable and can fragment into smaller, more than stable components. This is the footing of nuclear fission, or why uranium (diminutive number 92) and plutonium (atomic number 94) are used for atomic free energy (and nuclear weapons). And then over a big enough altitude, in that location's an limit to how much positive accuse you can put in the nucleus without making it highly unstable. This is why I wouldn't exist expecting to read much virtually the chemistry of the g-block elements anytime soon.
What Holds The Nucleus Together,
Source: https://www.masterorganicchemistry.com/2010/07/16/what-holds-the-nucleus-together/
Posted by: williamswict2001.blogspot.com


0 Response to "What Holds The Nucleus Together"
Post a Comment